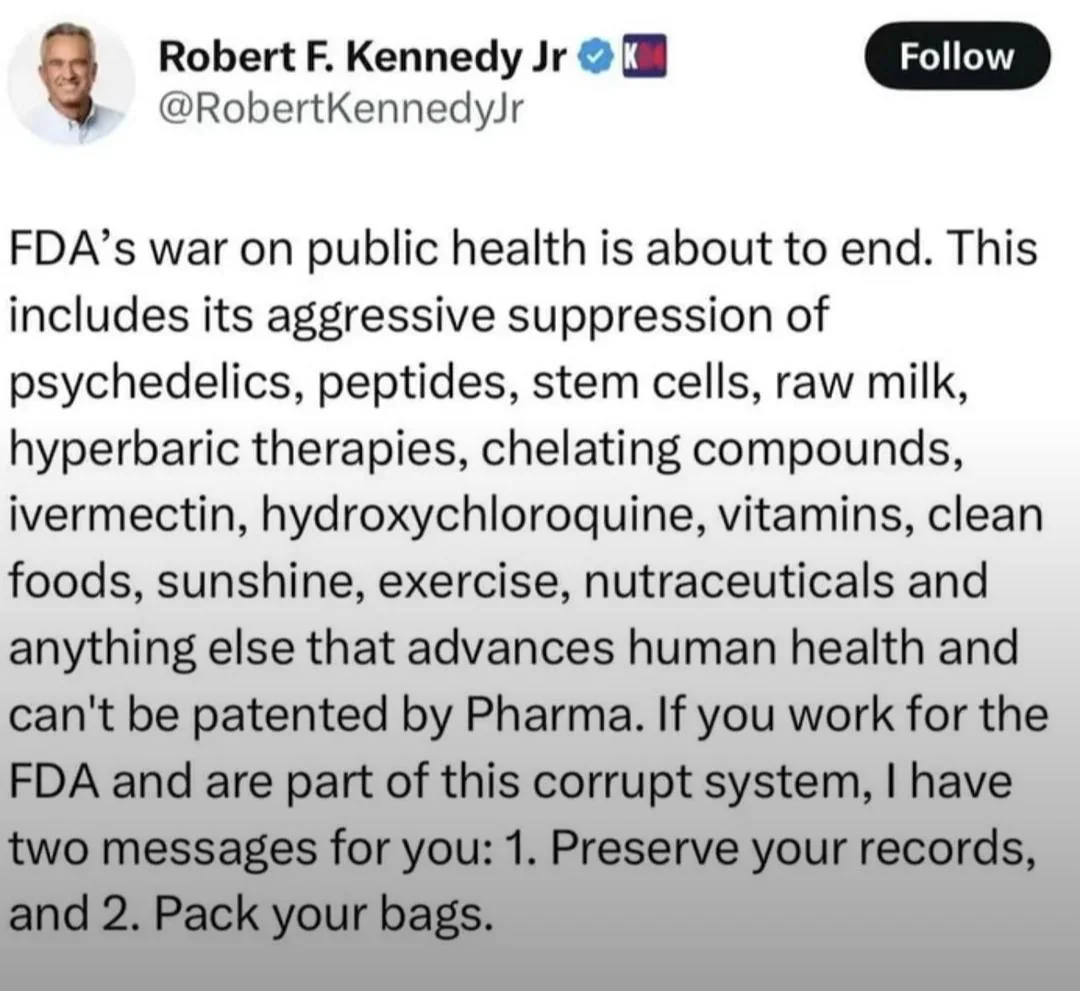
The FDA's policies have faced significant criticism for jeopardizing consumer safety. For instance:
Drug Approval Issues: Fast-track approvals have introduced drugs with limited evidence of safety, such as Aduhelm for Alzheimer's, which showed little benefit but posed risks. Harmful drugs are often slow to be withdrawn from the market.
Food Safety Loopholes: The GRAS designation allows companies to self-certify food additives without FDA oversight. Substances like potassium bromate, linked to cancer and banned internationally, remain in U.S. foods due to regulatory gaps.
Industry Influence: Close ties between FDA leaders and corporations raise concerns about conflicts of interest and impartiality in decision-making.
Weak Post-Market Oversight: The FDA lacks resources to monitor and act on safety issues after products are on the market, leaving harmful substances in circulation.
Some decision-makers should be held accountable for actions or policies that have negatively impacted public health and safety. A comprehensive overhaul of the food system is urgently needed to prioritize transparency, improve nutritional standards, and ensure consumer well-being.