Just one sample haha. Was trying to do it quick to not to get fired. I’m not too worried about problems with homogeneity since the powder appeared to be that of a pure compound (fine, white powder).
DISCLAIMER: This is not medical advice, engage in DIY HRT at your own risk; the intent of this post is harm reduction. This information is not intended for minors; by reading further you agree you are over the age of 21.
DISCLAIMER 2: This is not rigorous scientific research. I conducted this experiment over the course of a work day, carrying out my actual work while trying to not arouse suspicion. I did not have time to create a calibration curve to conduct quantitative analysis. Further, isolating samples and using IR and NMR to identify impurities would increase my chance of being found out by coworkers. I also forgot to collect a data point and made another mistake that I explained in the “results” section that I did not have time to correct. That being said, these results are roughly accurate and I am using them to inform my own DIY HRT.
This is somewhat of a follow-up of this post on DragonOrdnance estradiol enanthate, and I plan on posting every year with stability data for prepared solutions when properly stored (cool, dark, dry). Since I have 10 vials with 7 mL each, I have enough to provide over a decade of stability data. I also have leftover powder which I also plan on testing yearly.
Abstract:
To improve the safety of the DIY transfem community, it is necessary to demonstrate the thermal stability of estradiol esters to ensure DIYers properly sterilize injection vials. Without an autoclave, a higher temperature is necessary to destroy all bacterial endospores that may be present. Estradiol enanthate (EEn) has considerable thermal stability and can be sterilized at home without the fear of decomposing the EEn. At the suggested home-sterlization conditions of 130degC for 30 minutes, no decomposition was detected. Even at 180degC for 1 hour, less than 5% of the EEn decomposed into an unidentified compound. Light exposure also has minimal effect, with no decomposition detected after six months of continuous exposure to artificial light and indirect sunlight. Home sterilization is recommended to avoid infection. However, it is recommended to store both EEn powder and EEn solutions in a cool, dry, dark area to minimize the decomposition of EEn over longer periods of time (EEn powder is shelf-stable for five years and likely much longer). A follow-up study will focus on long-term stability of properly stored EEn powder and EEn injection solutions.
Introduction:
Last week, I saw a post about a comrade who started HRT and mentioned their sterilization method (130degC for 30 minutes). A discussion in the comments included users suggesting that estradiol enanthate (EEn) would start to decompose at 130degC. This surprised me, as I remember seeing somewhere that estradiol ester injection solutions can be sterilized at higher temperatures than an autoclave (121degC) without significant decomposition. This is important because autoclaves are effective not only for the high temperature, but for the elevated pressure as well (~30 psi, or ~2 atm). Therefore, slightly higher temperatures are required to effectively sterilize injection vials. DIYers likely don’t have access to an autoclave or any kind of pressure vessel, so to improve the safety of transfem DIYers, I aim to alleviate concerns regarding the thermal stability of EEn.
I have a considerable stash of EEn powder that I purchased 1.5 years ago (likely over 2 years past its manufacture date) and I have an injection solution I prepared in bulk, originally intending to prepare vials as needed. This bulk solution has not been subjected to heat but has been on my desk at home where it’s been subjected to artificial light and indirect sunlight (light, like heat, accelerates decomposition of EEn). In this experiment, I determined the stability of EEn under various conditions: as a powder and a solution, and after exposure of the solution to light and/or heat.
Methods:
All injection solutions were prepared as 50 mg/mL EEn in MCT oil with 2% (v/v) benzyl alcohol.
The EEn powder, MCT oil, and benzyl alcohol were kept at room temperature in opaque, resealable plastic bags since receiving them 1.5 years ago. The light-exposed injection solution was kept at room temperature in a clear, resealable plastic bag where it was continuously exposed to artificial light and indirect sunlight for 6 months. Two more injection solutions were prepared the same day as this experiment to represent injection solutions that have not been exposed to significant light.
The “light-exposed solution” was analyzed by GC-MS. Then, the solution was sealed in injection vials and the vials were placed in a GC oven at 130degC for 30 minutes. This “light-and-heat-exposed solution” was analyzed by GC-MS.
One of the freshly-prepared solutions was sealed in injection vials and placed in a GC oven at 130degC for 30 minutes. This is the “heat-exposed solution”. The other freshly-prepared solution was transferred to an open test tube and subjected to a 180degC oil bath for 1 hour. This is the “high-heat-exposed solution” and represents the worst-case scenario of a DIYer sterilizing at a temperature far beyond what is necessary.
Results/Analysis:
The chromatogram of the EEn powder suggests this is a high purity sample, as the only peaks are EEn (at 26.4-26.9 minutes) and the MCT oil from previous analyses (Image 1) This EEn powder was analyzed following the analysis of several EEn solutions, and the needle wash was empty, so there were three other peaks that were confirmed to be triglycerides from the MCT oil residue on the needle. There is also a solvent peak (acetonitrile) at ~1-2 minutes. The mass spectrum is consistent with that of EEn (Image 2).
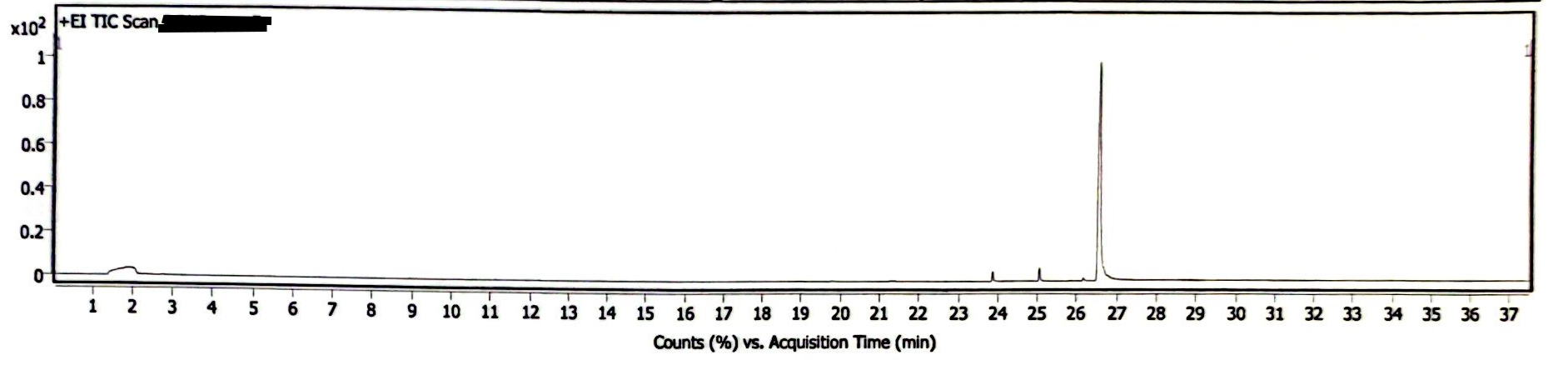
Image 1. GC Chromatogram of EEn powder
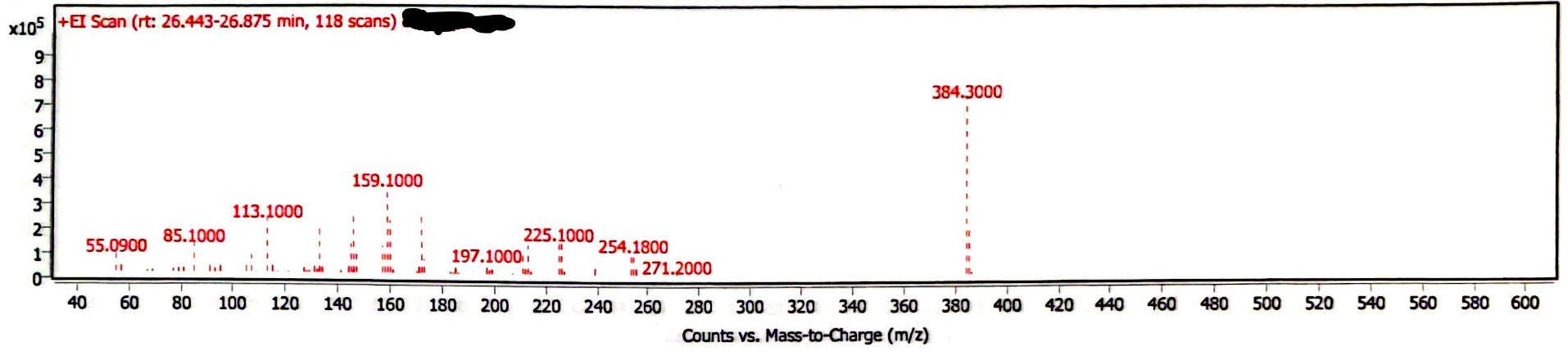
Image 2. Mass spectrum of EEn powder
Image 3 demonstrates a newly prepared EEn solution that was exposed to light and atmosphere while being heated at 180degC for 1 hour. Samples were diluted by adding 1 gram acetonitrile for every 10 mg of sample. As the samples were at the same concentration, the peak area of the EEn peak is a reasonable (though rough) measure of the remaining EEn. Before heating (t= 0), the peak area was 186.0 M counts. At t= 15 min, peak area was 184.9 M counts. At t= 30 min, peak area was 178.7 M counts. At t= 1 hr, peak area was 195.4 M counts. The peak area at 1 hr had the highest z-score at z= 1.5324, giving a p-value of .1254. This indicates it is very likely that the variations in these measurements are due to chance. The chromatogram at t= 1 hr demonstrates the production of a decomposition product, but the peak area of this decomposition product is <5% of the area of the EEn peak (Image 4).
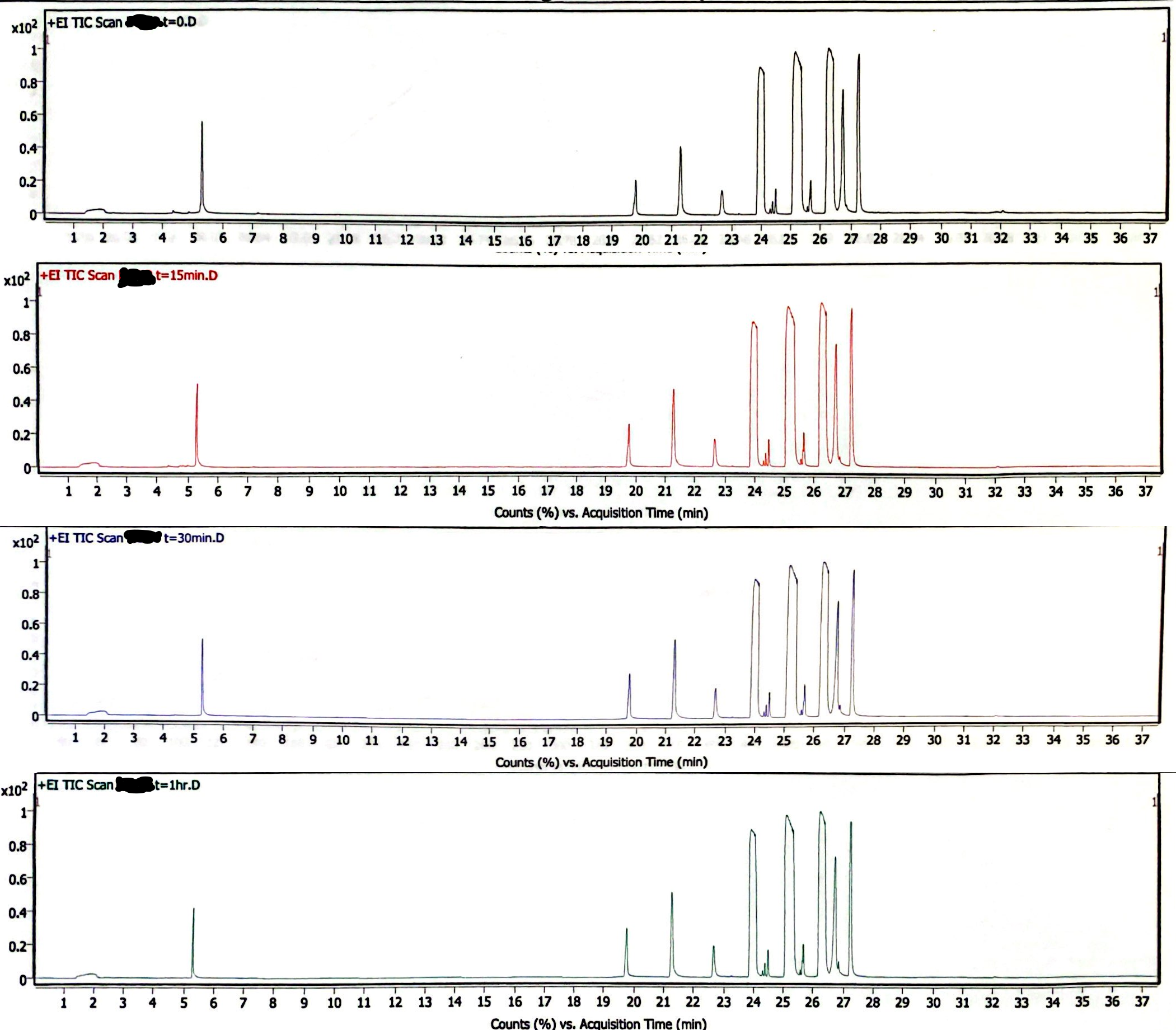
Image 3. High-heat solution chromatograms
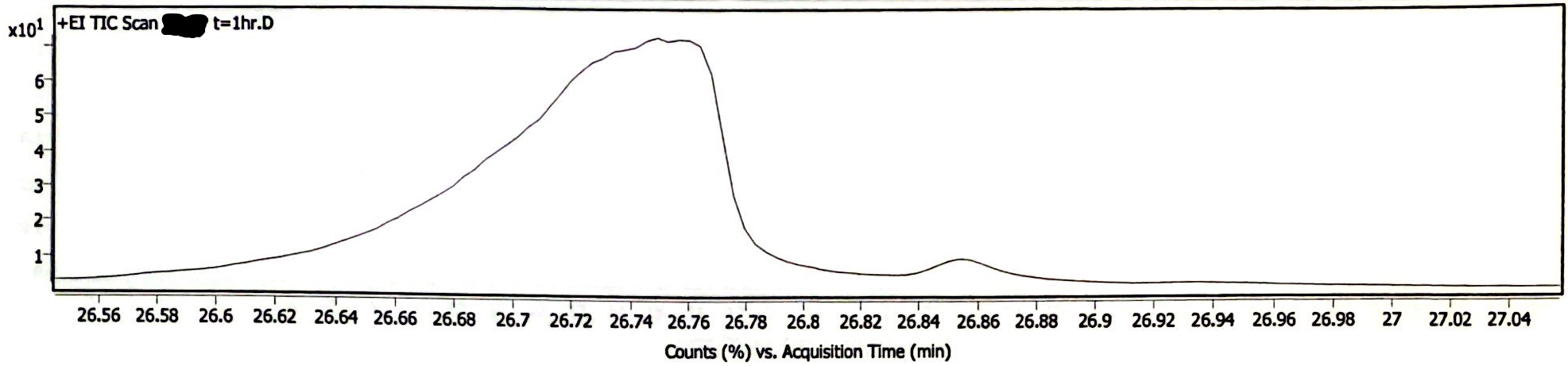
Image 4. High-heat solution chromatogram at t= 1hr, zoomed-in to visualize decomposition product peak
The following three chromatograms represent the light-exposed solution that was not heated (Image 5), the light-exposed solution that was heated at 130degC for 30 minutes (Image 6), and the newly-prepared solution that was heated at 130degC for 30 minutes (Image 7).
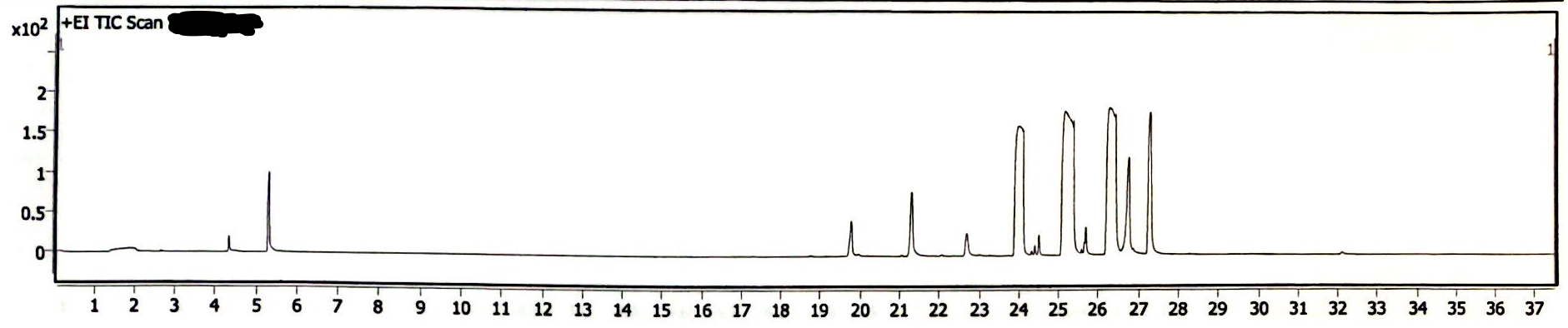
Image 5. Chromatogram of EEn solution exposed to light for six months
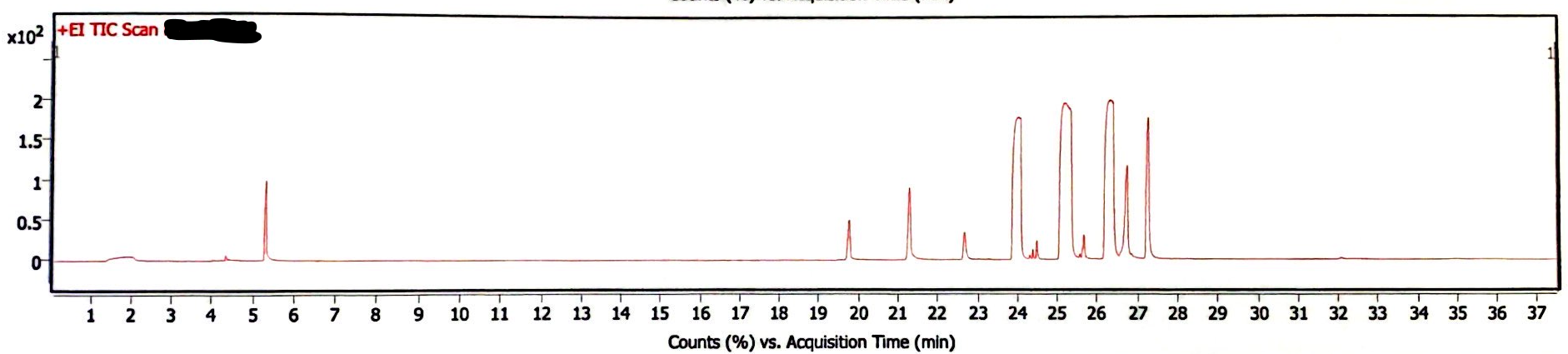
Image 6. Chromatogram of EEn solution exposed to light for six months and 130degC for 30 min
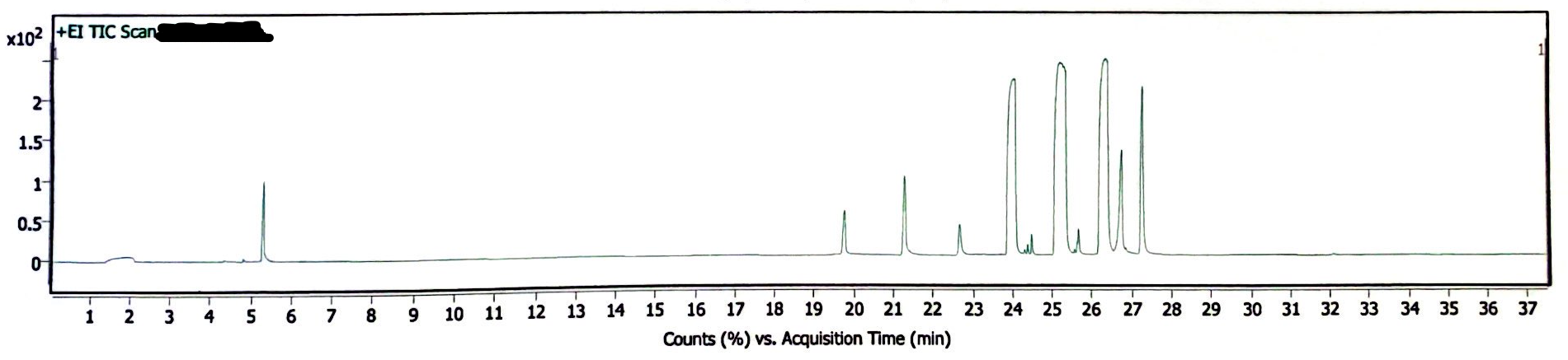
Image 7. Chromatogram of EEn solution exposed to 130degC for 30 min, but no light exposure
In absence of rigorous quantitative analysis, the proportion of peak area of EEn to benzyl alcohol was used to compare the decomposition of EEn. The concentration of benzyl alcohol in these solutions was constant, so it is another reasonable yet rough estimation of remaining EEn to determine if decomposition occured. For the light-exposed/no-heat solution, the EEn peak was 68.99% the area of the benzyl alcohol peak. For the light-exposed/heat-exposed solution, the EEn peak was 68.41% the area of the benzyl alcohol peak. For the no-light/heat-exposed solution, the EEn peak was 68.20% the area of the benzyl alcohol peak. These values are not significantly different, indicating that decomposition did not occur in any of these samples. (I forgot to measure the peak areas of a no-light/no-heat solution, I’m sowwy :(, but you can look at the 180degC data to see that no significant decomposition occurred at 30 minutes at such a high heat).
Conclusion:
According to GC-MS data, no significant decomposition at normal sterilization conditions (130degC for 30 minutes). Even at 180degC, EEn is fairly stable, remaining 95% intact after one hour at this extreme temperature. Light exposure is also a minimal risk, as no significant decomposition occurred after six months of moderate light exposure.
Thanks! I don’t have access to an autoclave but tbh you don’t really need to even boil them. The benzyl alcohol will kill anything in the solution except the endospores. The autoclave kills bacterial spores IIRC from my microbiology course, but the risk is very low for IM injections (not an expert on this so look into it further if you’re worried). Boiling will NOT kill endospores, so IMO, there’s no difference between boiling and just using benzyl alcohol.
DISCLAIMER: This is not medical advice, engage in DIY HRT at your own risk; the intent of this post is harm reduction. This information is not intended for minors; by reading further you agree you are over the age of 21.
DISCLAIMER 2: This is not rigorous scientific research. I conducted this experiment over the course of a work day, carrying out my actual work while trying to not arouse suspicion. That being said, I am using these results to inform my own DIY HRT.
Earlier this year, I bought estradiol enanthate (EEn) from Dragon Ordnance ( dragonordnance.io or dragonordnance.com ; .io seems to be down but they’re run by the same people). Shipping was expensive ($50 I think) because they are located in China, but the prices are low ($5 for a gram that will last over 2 years). You have to email them for information on how to send payment, either sending cryptocurrency or a cash transfer via the Wise app. The people are really nice too; the person processing orders had COVID when I ordered and my order was delayed a week or two so they sent me double my order. I’m sitting on a 25 year supply now so I’m going to be giving away a lot of E to friends.
I also bought MCT oil as a solvent for the injectable solution and benzyl alcohol as a preservative from Dragon Ordnance. I used a volumetric flask to make a 50 mg/mL EEn solution with 2% v/v benzyl alcohol, but if you don’t have access to volumetric flasks you can make a vial with 500 mg EEn, 0.2 mL benzyl alcohol, and 9.35 mL MCT oil. This wiki is a good resource for DIY MtF HRT, but the owner of the group is an outspoken anti-communist. I plan on injecting 0.3 mL (15 mg EEn) every 14 days, but this wiki recommends 0.22 mL (11 mg EEn) every 7 or 10 days. Use distilled water to determine which liquid is MCT oil and which is benzyl alcohol. The MCT oil will float on distilled water, while the benzyl alcohol sinks. Also, 1 mL of benzyl alcohol will dissolve in 25 mL of water if you mix it thoroughly, while MCT oil will not dissolve in water.
I happen to be a chemist who has access to a variety of analytical instruments so I ran UPLC to test for purity and GC-MS to verify that the chemical is actually estradiol.
The UPLC chromatogram indicates very high purity (>99%), as only one peak was visible.

The extracted UV-Vis spectrum of the UPLC at 8.429 minutes (the sole peak above) was consistent with the expected UV absorption of estradiol esters.
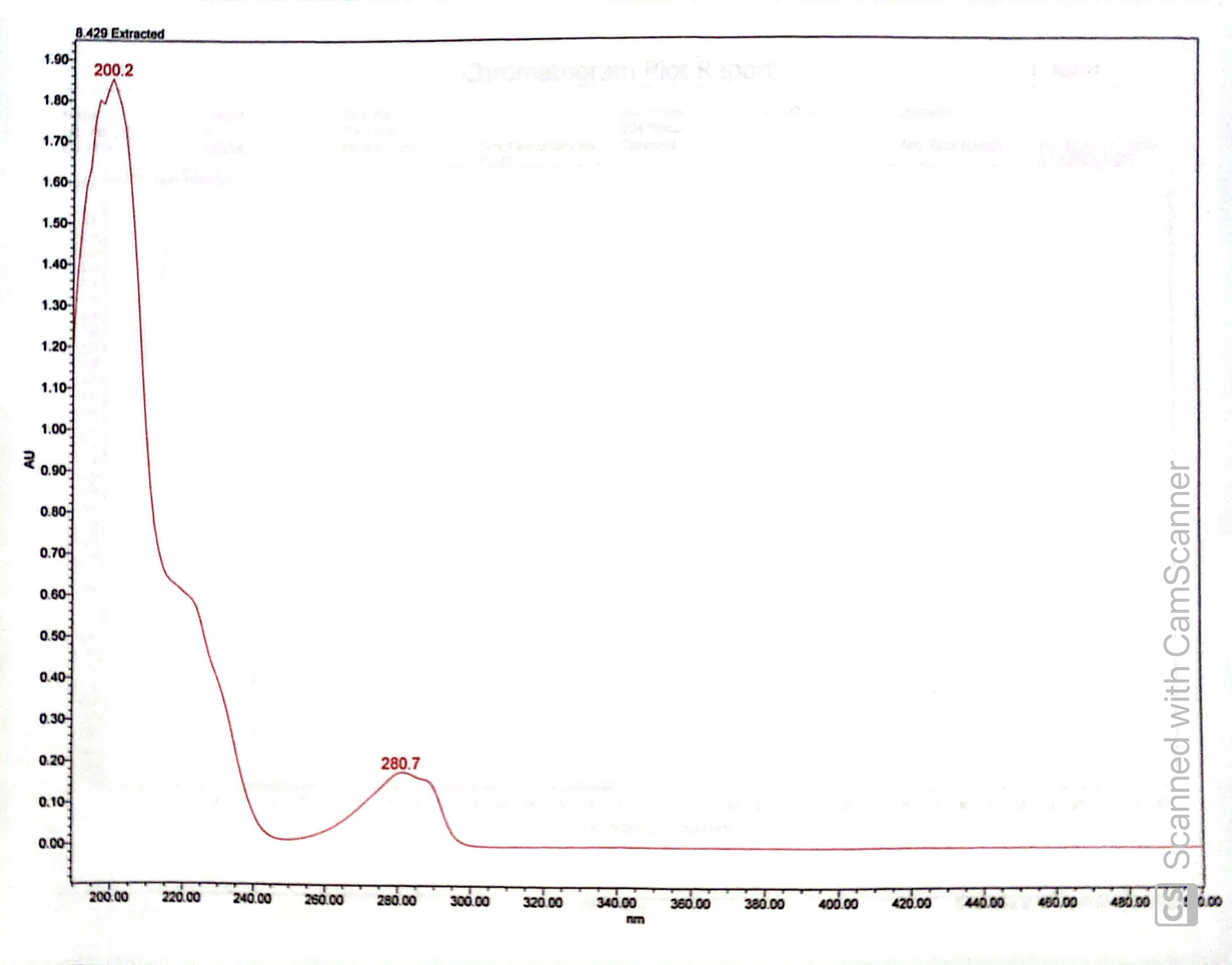
The GC-MS chromatogram similarly indicated the presence of just one compound at 26.628 minutes (the first peak is the solvent I used, and the following two peaks are solvents commonly used in our lab and are likely only present in this chromatogram due to residue on the sample injection needle). The small peak between 14 and 15 minutes is likely a breakdown product formed in the harsh GC conditions.
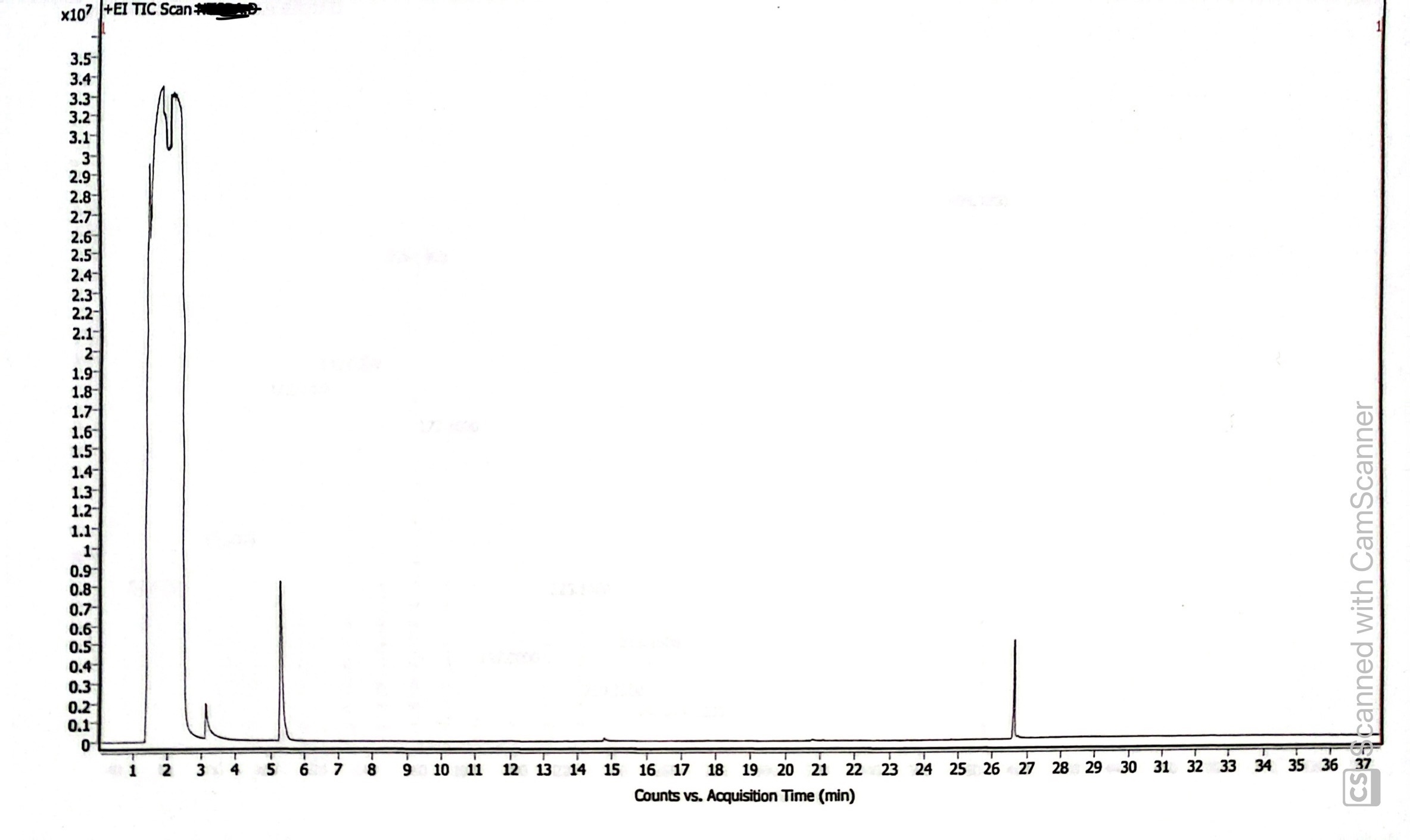
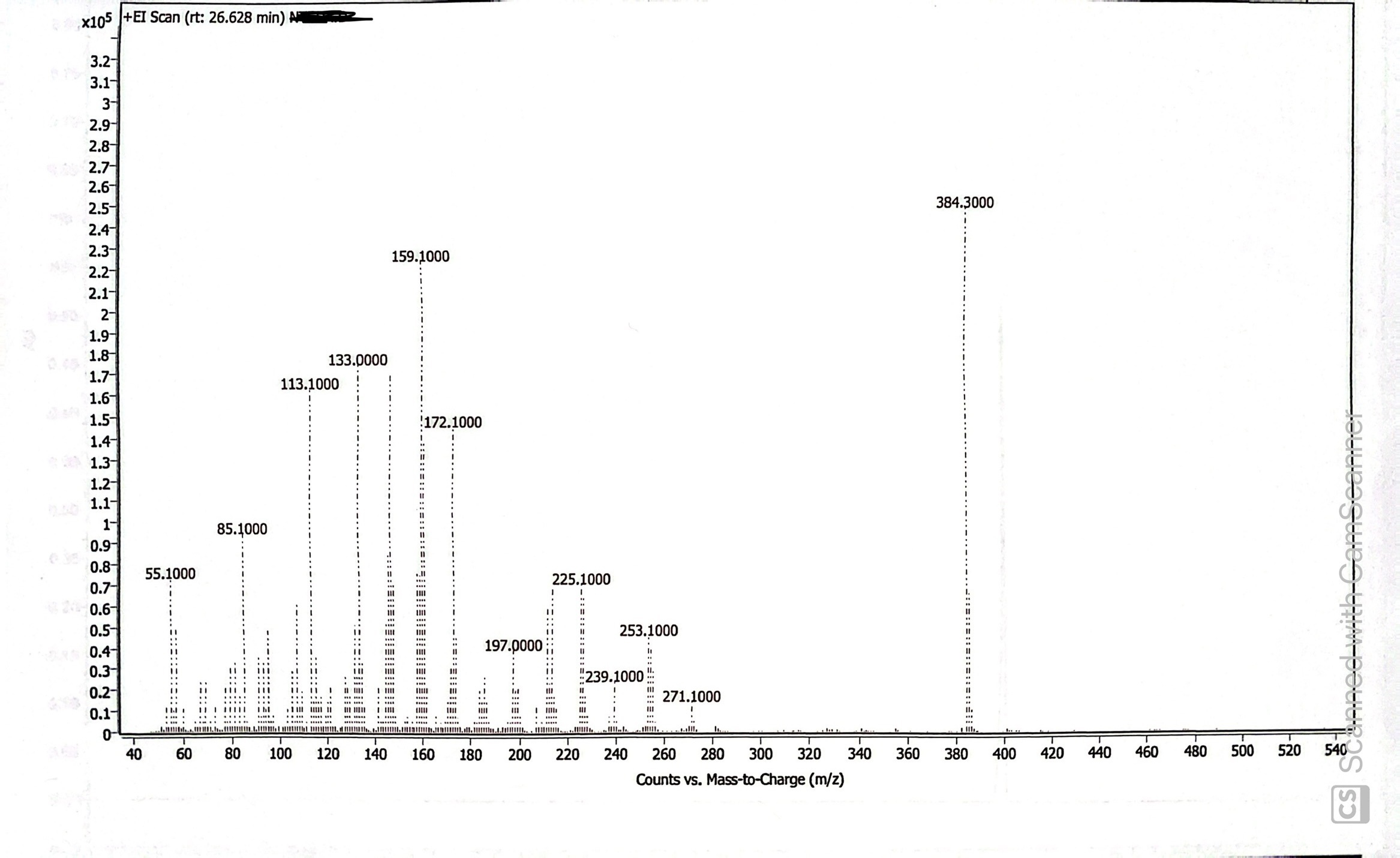
The mass spectrum at 26.628 minutes indicates the compound is estradiol enanthate. The NIST library we use indicated a >90% chance that the compound is some estradiol derivative based on the fragmentation. The base peak at 384.3 m/z is sufficient evidence to identify the compound as estradiol enanthate, which has a molar mass of 384.56 g/mol.
I am confident that the powder sent by Dragon Ordnance is high purity estradiol enanthate and would highly recommend this vendor for their low price, quality product, and good service.
Hey! Sorry, I haven't logged in on this account since I made this post. I would really appreciate it if you could share this with them. I am not comfortable doing so myself. Thanks!